NETWORK FOR THE EMPIRICAL STUDY OF TRIPS+ INTELLECTUAL PROPERTY, TRADE AGREEMENTS, AND ACCESS TO HEALTH PRODUCTS
An informal network formed at the Global Congress on Intellectual Property and the Public Interest in 2018 to advance the empirical study of the impact of TRIPS-Plus Free Trade Agreements (and FTA provisions) on access to pharmaceuticals, vaccines, and diagnostics. We run a series of occasional webinars and an email list. To join the email list, visit tinyurl.com/fme-subscribe
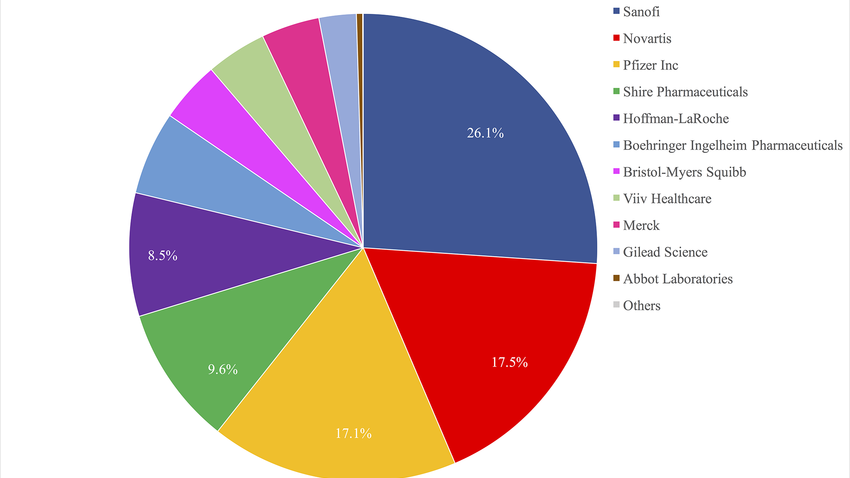
Presentation of the TRIPS-Plus PTA Dataset by Jean-Frédéric Morin
[November 15, 2020] The T + PTA dataset fills this gap by documenting the existence of 90 types of IP provisions in 126 agreements signed between 1991 and 2016. The dataset reveals that, even for like-minded countries, significant variations exist in their reliance on TRIPs-plus provisions, their degree of consistency across trade agreements, and their preferences for some IP rights. Flyer | Video

PRESENTATION OF THE MEDSPAL DATABASE BY AMINA MAILLARD, MEDICINES PATENT POOL
PANEL DISCUSSION ON the U.S.-MExico-Canada Agreement (NAFTA 2.0) AND ACCESS TO MEDICINES
Studies of the Impact of FTAs with TRIPS+ Provisions
- Danielle Trachtenberg, Warren A. Kaplan, Veronika J. Wirtz, and Kevin P. Gallagher. The Effects of Trade Agreements on Imports of Biologics: Evidence from Chile. Journal of Globalization and Development. Vol 10, No 2. (2020). Link.
- Boston Univeristy Working Group on Trade, Investment Treaties & Access to Medicines. (Lead Authors: Rachel D. Thrasher, Veronika J. Wirtz, Warren Kaplan, Kevin P. Gallagher, Hattie Werk). Rethinking Trade Treaties and Access to Medicines. (2019). Link.
- Ellen R. Shaffer and Joseph E. Brenner. A Trade Agreement’s Impact On Access To Generic Drugs. Journal of Health Affairs. (2009) Link.
- Rohit Malpani. All Costs, No Benefits: How the US-Jordan Free Trade Agreement Affects Access to Medicines. Journal of Generic Medicines. Vol 6, No. 3. (2009). Link.
Studies of the Impact of TRIPS+ Provisions Frequently Found in FTAs
Studies on the impact of secondary patents
- María José Abud, Bronwyn Hall, and Christian Helmers. An Empirical Analysis of Primary and Secondary Pharmaceutical Patents in Chile. PLoS ONE. (2015) Link.
- Amy Kapczynski, Chan Park, and Bhaven Sampat. Polymorphs and Prodrugs and Salts (Oh My!): An Empirical Analysis of “Secondary” Pharmaceutical Patents. PLoS ONE. (2015). Link.
- I-MAK. Overpatented, Overpriced: How Excessive Pharmaceutical Patenting is Extending Monopolies and Driving up Drug Prices. White paper. (2015). Link.
Studies on the impact of data exclusivity
- Michael Palmedo. Evaluating the Impact of Data Exclusivity on the Price per Kilogram of Pharmaceutical Imports. GEGI Working Paper no. 48. Boston University Global Development Policy Center. (April 2021). Link.
- Miguel Ernesto Cortés Gamba, Francisco Rossi Buenaventura, and Mayra Damaris Vásquez Serrano. Impacto de 10 Años de Protección de Datos en Medicamentos en Colombia. Serie Buscando Remedio No. 2. (2013). Link
- Aaron S. Kesselheim and Daniel H. Solomon. Incentives for Drug Development — The Curious Case of Colchicine. New England Journal of Medicine. (2010). Link.
Studies on the impact of Linkage
- Ron A. Bouchard, Richard W. Hawkins, Robert Clark, Reider Hagtvedt, and Jamil Sawani. Empirical Analysis of Drug Approval-Drug Patenting Linkage for High Value Pharmaceuticals. Northwestern Journal of Technology and Intellectual Property. Vol. 8 No. 2. (2010). Link.
Studies on the impact of TRIPS+ enforcement
- John Zarocostas. Brazil and India file complaint against EU over seizure of generic drugs. British Medical Journal, 340. (2010). Link.