Professor Brook K. Baker
Senior Policy Analyst Health GAP
June 6, 2021
The complications and limitations of compulsory-license-reliant measures to respond to the COVID-19 pandemic need to be better explained. The European Union and several other countries espousing reliance on TRIPS-compliant compulsory licenses to overcome patent barriers have opposed the India/South Africa temporary intellectual property (IP) waiver proposal on COVID-19 health technologies at the World Trade Organization. Although compulsory licenses (CLs) on patent alone may be sufficient to allow generic production of small molecule medicines, CLs are unlikely to suffice with respect to vaccines, biologic medicines, including monoclonal antibodies, and more complex diagnostic tests, medical devices, and respirators. TRIPS-compliant compulsory licenses do not reach the additional IP rights covered by the waiver – confidential information/trade secrets, regulatory data, copyright, and industrial design, each of which plays a critical role in blocking alternative producers from manufacturing IP-protected health technologies. Even with respect to patents alone, country-by-country and product-by-product compulsory licenses are ill-suited to amassing the necessary components for vaccine manufacture. The explanation below outline the deficiencies of the EU’s proposals to rely on CLs even to override patents.
Complex supply chains require dozens of CLs to gain access to key components
Patent rights give rightholders the power to exclude competition – to bring a private infringement claim against alternative manufacturers to prevent them from “making, using, offering for sale, selling, or importing” the infringing product or product produced by an infringing process (TRIPS Art. 28). Countries can also enlist border measures to prevent the export, transshipment, or importation of patent infringing products (TRIPS Arts. 51-60).
The International Federal of Pharmaceutical Manufacturers and Associations (IFRMA) like to explain the complexities of the supply chain for messenger RNA (mRNA) and viral vector vaccines: “Vaccine supply chains are international. The BioNTech/Pfizer vaccine contains 280 ingredients sourced from 19 countries. Moderna’s AstraZeneca’s and Johnson & Johnson’s are similarly complex.” This vey complexity is part of what make compulsory licensing the wrong tool to respond to the need to increased production of COVID-19 vaccines.
Basically, for each and every patented component or process where the patent-owner was not willing or able to sell sufficient quantities and not willing to grant a voluntary license, the final destination importing country that wishes to rely on CLs would need to find another producer willing to apply for a CL for that component (in the case of an all sector commercial CL) or accept a non-commercial/ government use license for that component. The final destination country would need to convince the country where the component licensee was manufacturing to issue the requisite CL for export.
Making the process even more complex, if the next stage of the vaccine manufacturing process needs to take place in another country besides the final destination importing country – let’s call this the “formulation” country, the final destination country would have to convince the formulation country to issue both an import license for each patent-blocked component and for the vaccine itself and an export license for the formulated vaccine. At the formulation stage for mRNA vaccines, for example, licensed manufacturers need complex machinery and processes to combine patent-protected mRNA, protein caps, and lipid nanoparticles, and various stabilizers. Having issued multiple import CLs to allow the formulator to amass needed inputs, the country where formulation was undertaken would have to also issue an export license so the formulation could go to the next stage, say fill and finish. If the fill and finish stage too cannot be accomplished locally in the final destination country, another round of import and export licenses must be granted by the fill-and-finish country.
As a final stage, the country of final destination and use would itself have to issue a CL for import.
If countries involved in this complex chain of events were relying on Article 31bis, appropriate notifications to the WTO would be required by all the exporting and importing countries in the chain.
In sum, at least for CL-compliant vaccine manufacture that is not “vertically integrated” (all phases in a single plant), a harrowing number of CL must be coordinated and granted in multiple countries.
The patent landscape on COVID-19 is extensive and complex
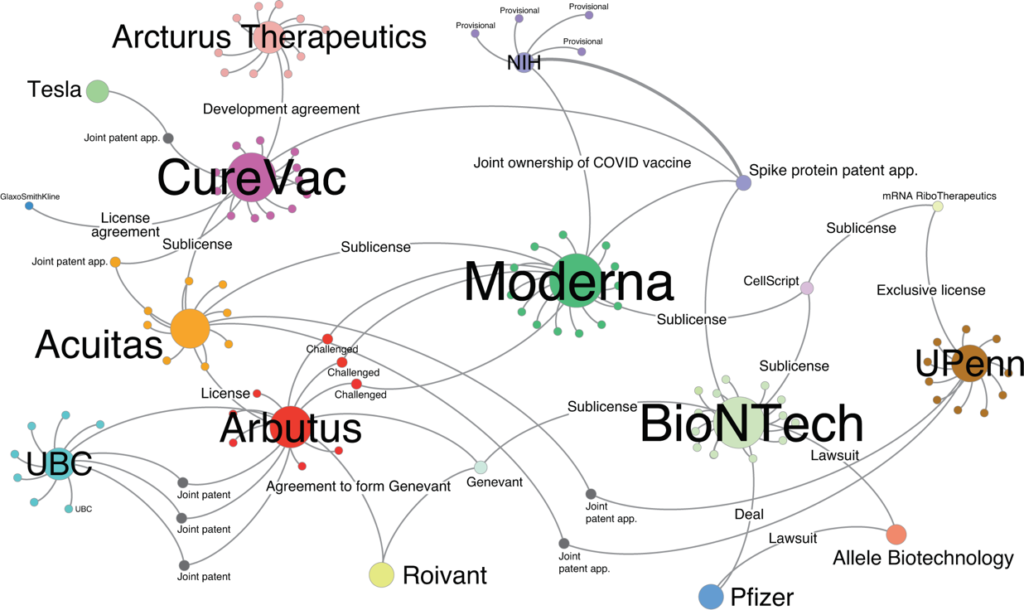
The scenario described above assumes that the components and products are all patented in the country of manufacture/export and import/use. That may or may not be true, but we are still discovering how extensively companies have been patenting. Early reports are that there is a complex web of patent on COVID-19 vaccine technologies and that they have been filed in many countries capable of producing components and vaccines (additional source).
Figure from A Network Analysis of COVID-19 mRNA Vaccine Patents
Large nodes represent the relevant entities while the edges represent agreements or patents between two entities. Smaller nodes around the entities represent patents that were identified as being relevant to the underlying vaccine technology
CL complications multiply further
Even what described above might be further complicated by the fact that countries in the described chain have not yet adopted Article 31bis export/import CL rules. TRIPS Article 31bis is not self-implementing in countries, nor would the EU’s proposed CL clarifications be self-implementing. Even if all countries in the supply chain were to adopt Article 31bis rules, each supplying/exporting country would have to cooperate. One break in the chain of needed CLs could scuttle the whole effort.
As emphasized in the introduction, CLs on patents would not be enough. To enable production of bio-identical vaccines, each country that needed IP protected confidential information, trade secrets, regulatory data, copyright protected software, blueprints, and papers, and industrial designs would have to enact a sui generis set of rules over-riding these multiple IP protections. They would also have to navigate how to force technology transfer for confidential information, trade secrets, and biologic resources that are held in other countries.
The CL complexities described above apply to each country that requires vaccines. Given that low- and middle-income countries currently lag way behind given corporate and rich country collusion to prioritize the Global North, a large number of countries might need to go through this same elaborate and confusing CL labyrinth. All of this red tape could be removed if WTO adopts the TRIPS waiver and countries quickly follow suit domestically.
Europe pretends that these problems of notification, cooperation, and coordination don’t exist and that the existing TRIPS flexibilities are sufficient. They aren’t and the EU’s four proposed CL clarifications offer nothing of substance. CLs for patents demonstrably insufficient to allow scale-up production of COVID-19 vaccines to save lives, avoid continuing social and economic chaos, and prevent emergence of new variants that can undermine existing immunity. It’s time to put the false idea that TRIPS flexibilities on their own are equal to the task. The EU and other waiver-blocking countries must face the facts that IP barriers must be removed if we are to survive this viral plague.