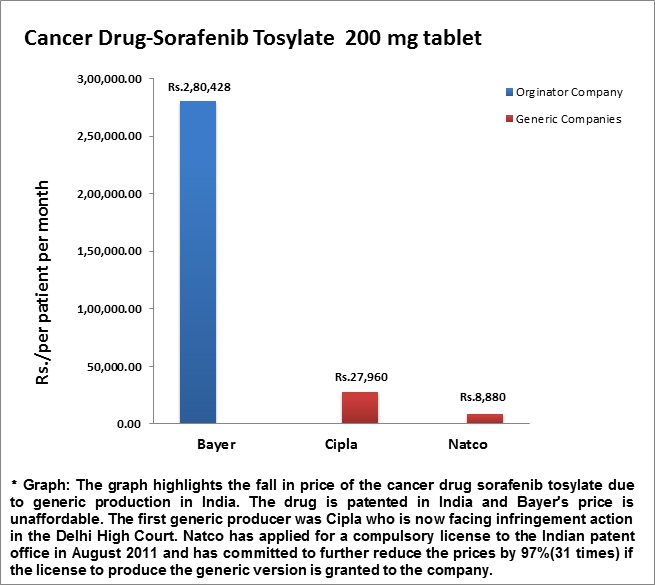
The Indian pharmaceutical company Natco has applied for a compulsory license for sorafenib, an anticancer drug sold by Bayer under the brand name Nexavar. The price charged by Bayer is Rs 2,80,428 per month (USD 5,600), a price unaffordable to the majority of Indians in need of the drug (the compulsory license application shows that very few people in need of the medicine receive it). Cipla produced a generic version of the drug which it sold for a tenth of Bayer’s price, but it now faces an infringement action. Natco intends to sell the drug for even less if it receives the compulsory license.
The Indian pharmaceutical company Natco has applied for a compulsory license for sorafenib, an anticancer drug sold by Bayer under the brand name Nexavar. The price charged by Bayer is Rs 2,80,428 per month (USD 5,600), a price unaffordable to the majority of Indians in need of the drug (the compulsory license application shows that very few people in need of the medicine receive it). Cipla produced a generic version of the drug which it sold for a tenth of Bayer’s price, but it now faces an infringement action. Natco intends to sell the drug for even less if it receives the compulsory license.
In a recent hearing at the Patent Office, Bayer argued that it could not sell the drug at a lower cost because in needed to recoup research and development costs. Bayer was then asked to disclose the amount of money it spent on R&D for the drug.
For more information, see:
- Natco’s application for a compulsory license.
- Update on first Compulsory License application in India for cancer drug – Sorafenib. January 17, 2012.
- CH Unnikrishnan for Live Mint. Bayer Asked to Give Cost Data. January 13, 2012.
- Update on CL application on cancer drug sorafenib tosylate. November 22, 2011.
Branded v. Generic Prices for Sorafenib Tosylate (Source: donttradeourlivesaway.com)